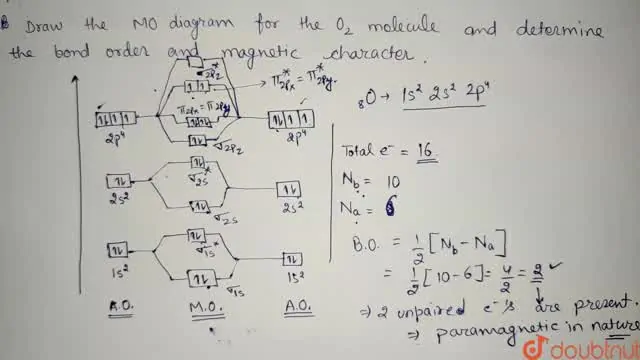
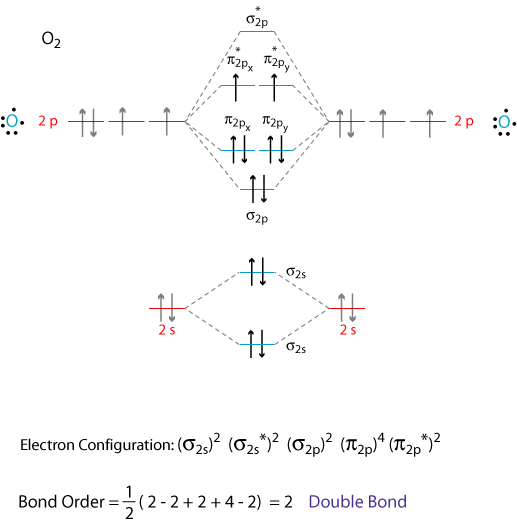
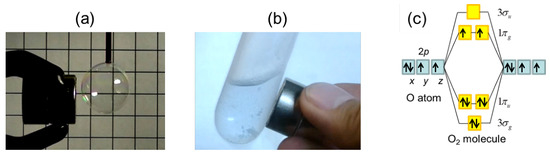
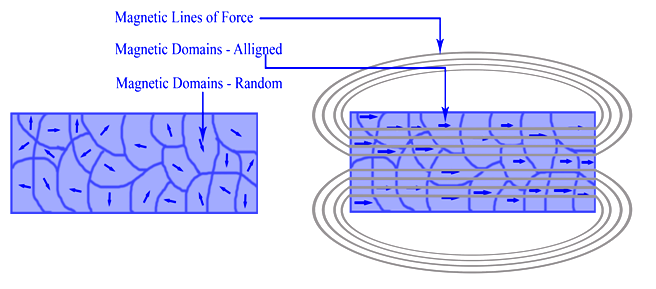
Write the molecular orbital electronic configuration of N2 and O2 molecules with the of molecular orbital theory. Predict its magnetic behaviour also.

Schematic of the 'O2' molecular orbital diagram. The figure explains... | Download Scientific Diagram
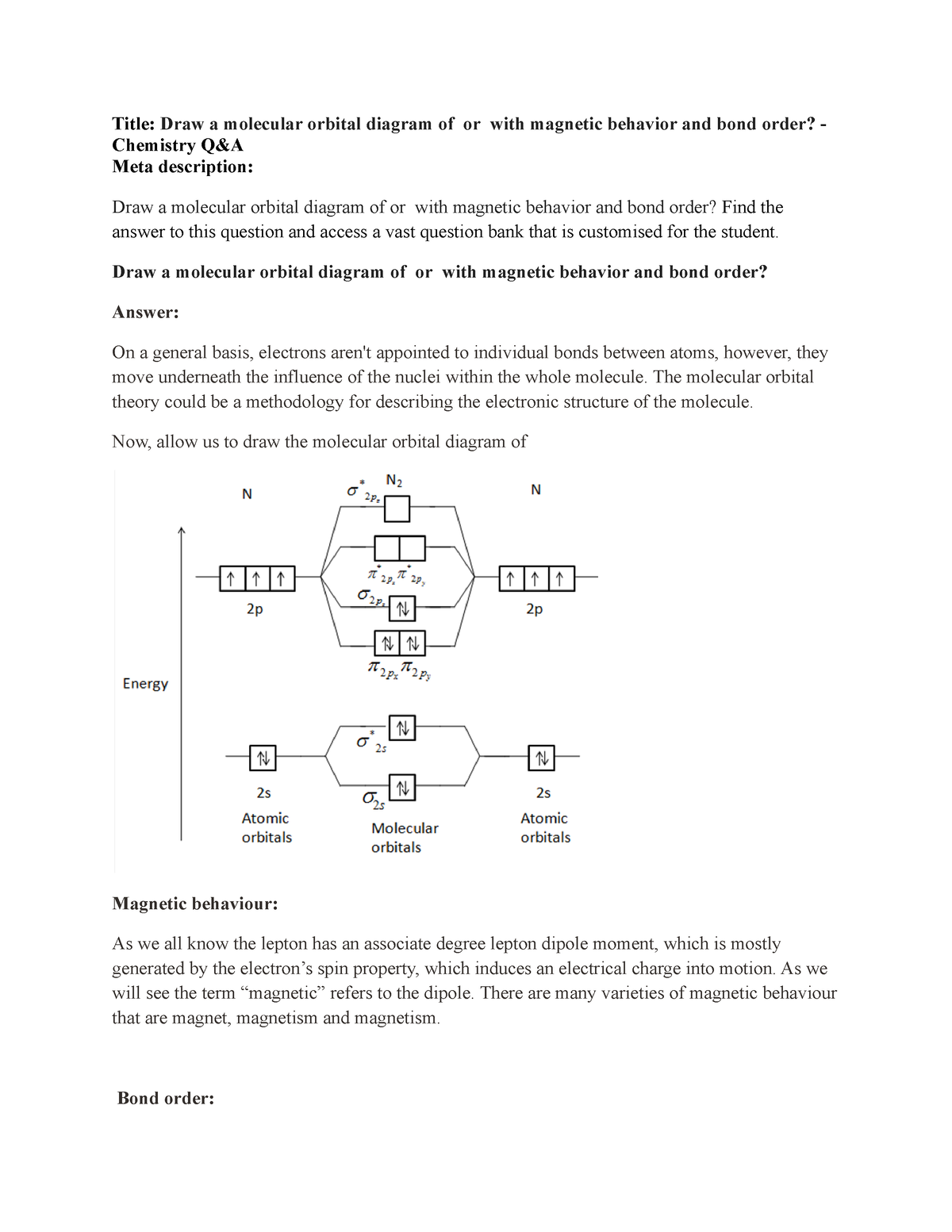
22-Draw a molecular orbital diagram of ${N 2}$ or ${O 2}$ with magnetic behavior and bond order - Studocu

Molecular Orbital Theory || MOT || BMOs and ABMOs|| Bond Order || Magnetic Behaviour|| H2 Formation - YouTube
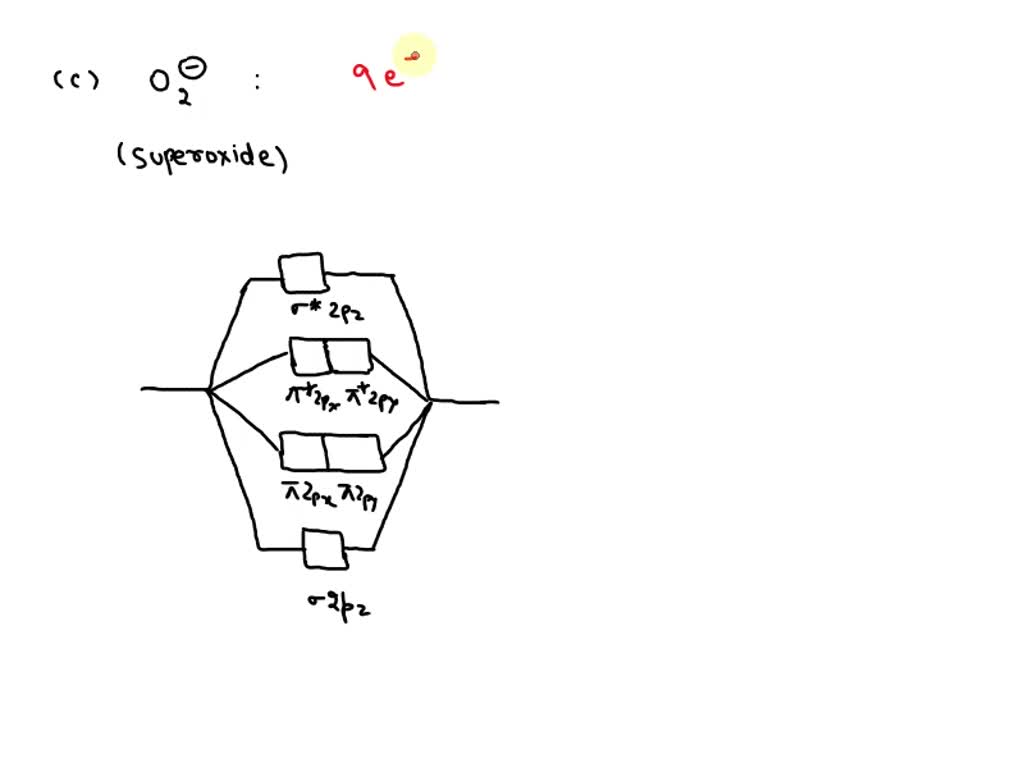
SOLVED: Compare the relative stability of the following species and indicate their magnetic properties (that is, diamagnetic or paramagnetic): O2, O2^+, O2^- (superoxide ion), O2^2- (peroxide ion).