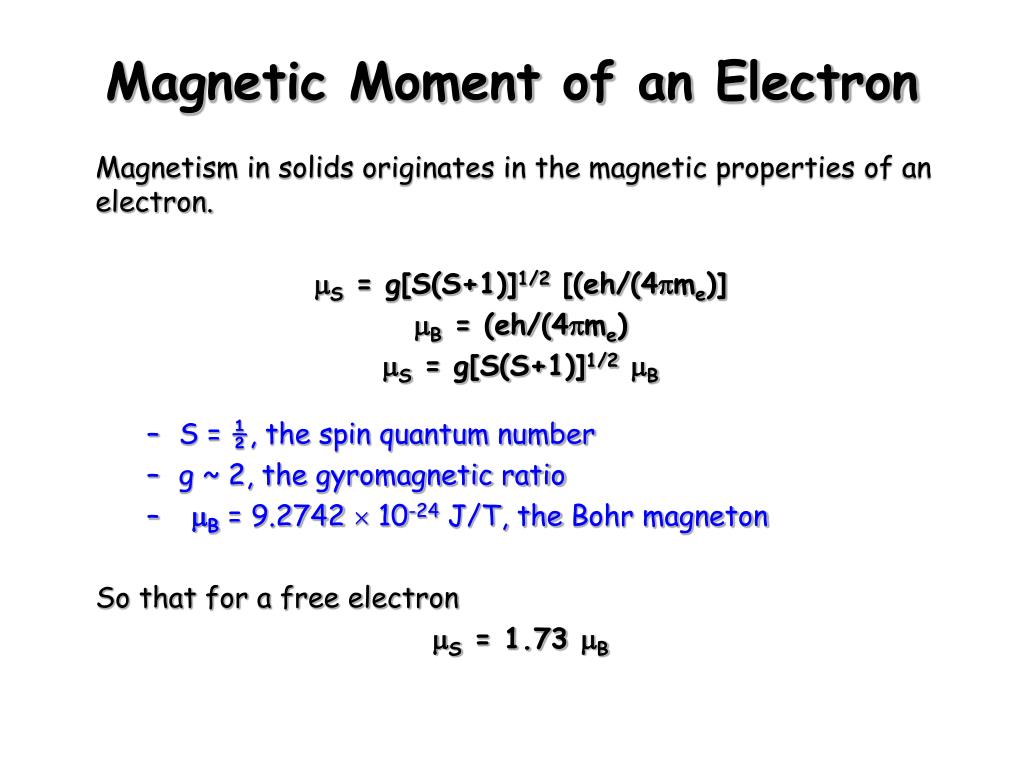
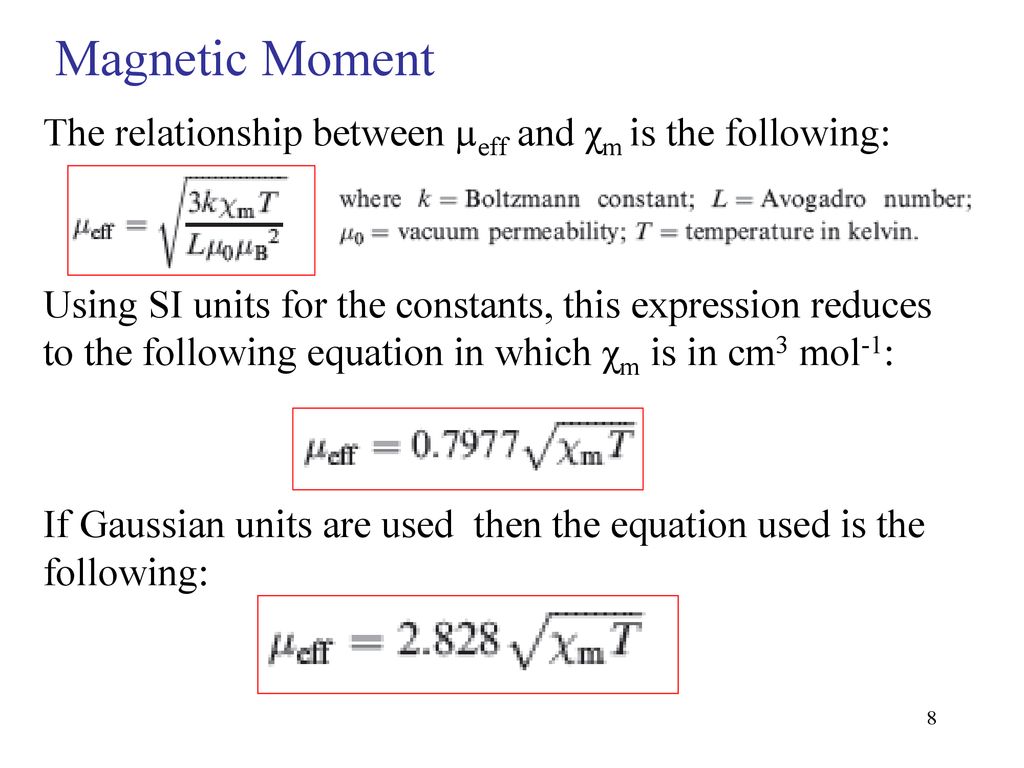
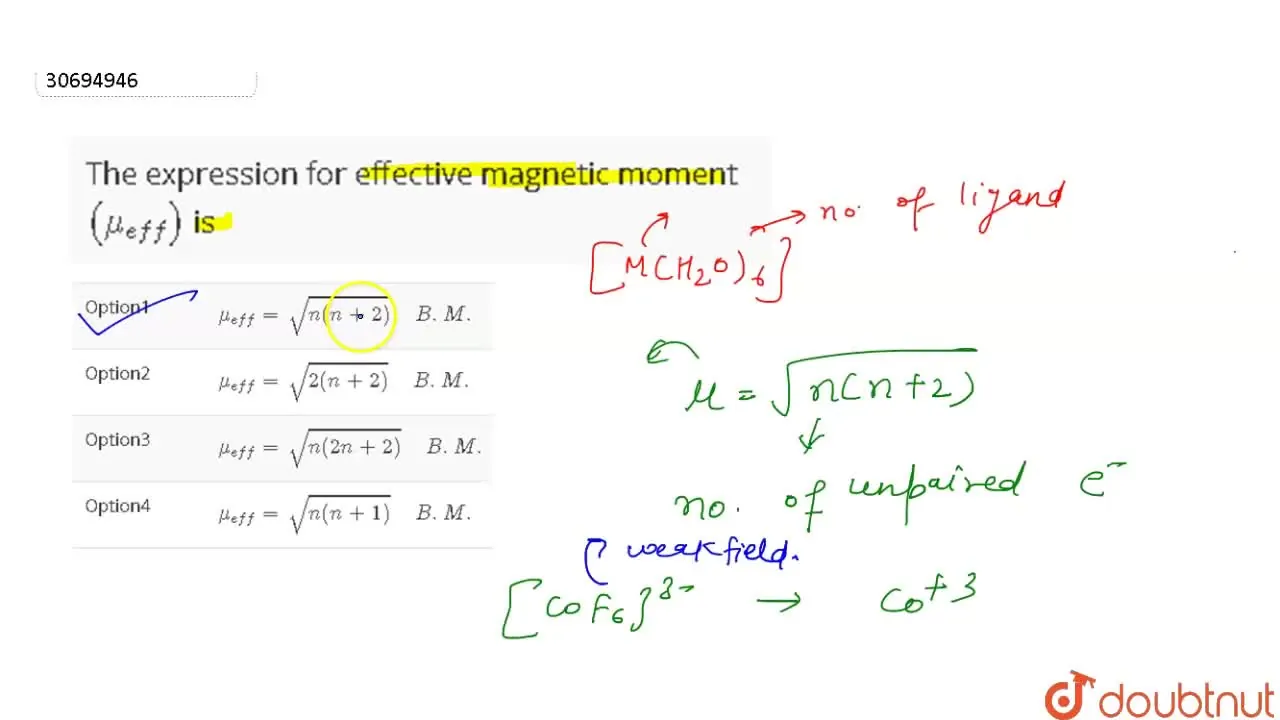
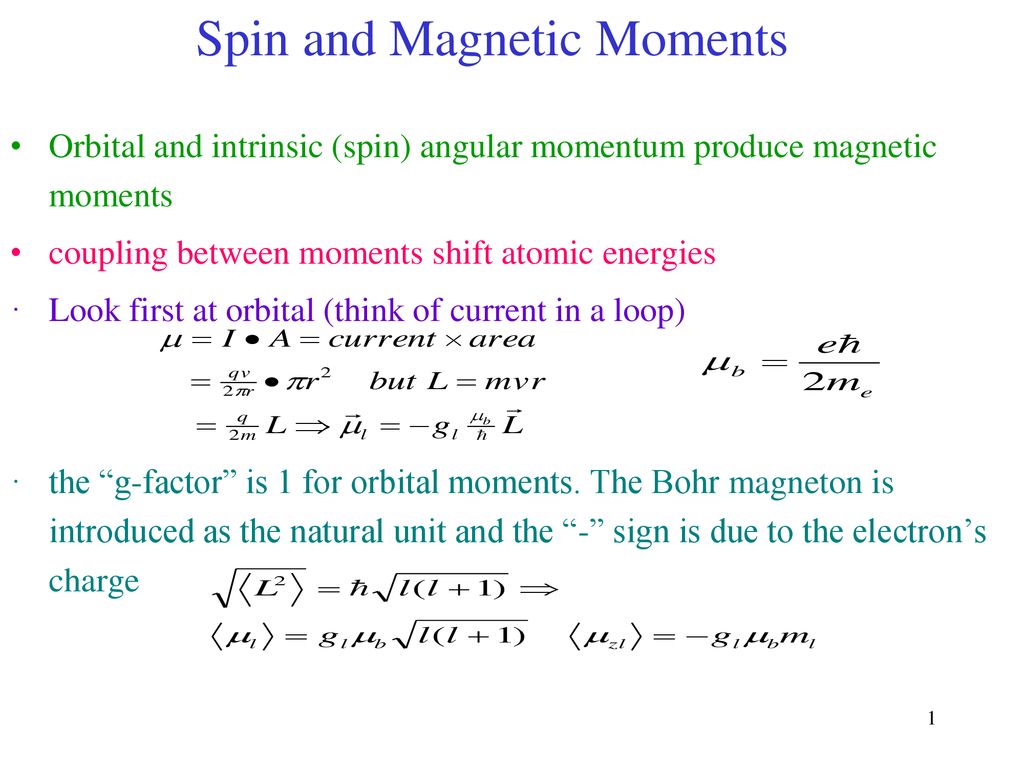
The expression for effective magnetic moment \( \left(\mu_{\text {eff. }}\right) \) is: (a) \( \... - YouTube
The magnetic moments of two bar magnets of same size are in the ratio 1:2 when they are placed one over the other with theirsimilar poles together then their period of oscillation

Temperature dependence of the effective magnetic moment (calculated... | Download Scientific Diagram
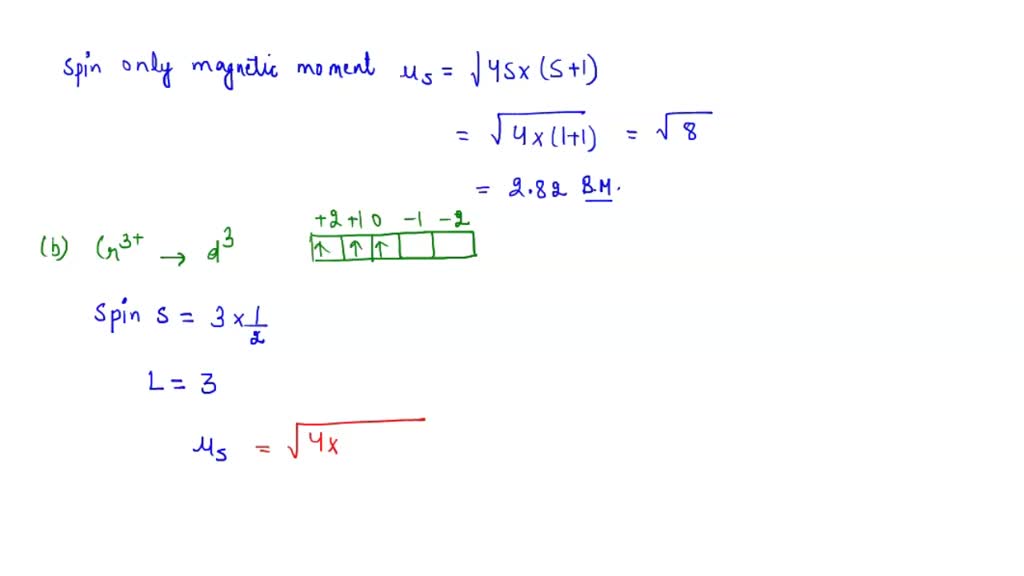
SOLVED: Calculate the magnetic moment of V3+, Cr3+, Pr3+, and Nd3+ according to the following instructions: (a) Consider the spin-only magnetic moment in your calculation. (b) Consider both the spin and orbital

The effective magnetic moments of Co2+ and Co3+ in SrTiO3 investigated by temperature-dependent magnetic susceptibility - ScienceDirect

Calculated effective magnetic moment µ eff (calc) of rare-earth ions,... | Download Scientific Diagram
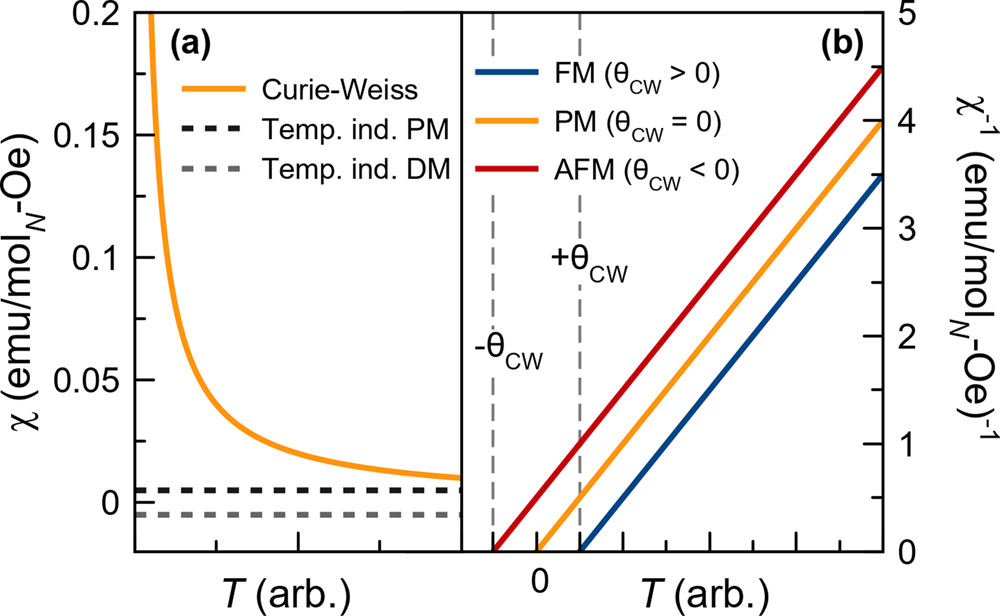
Tutorial: a beginner's guide to interpreting magnetic susceptibility data with the Curie-Weiss law | Communications Physics

Calculate the magnetic moment of an atom (in Bohr magnetons) (a) in ^{1}F state;(b) in ^{2}D_{3/2} state;(c) in the state in which S = 1 , L = 2 and L and

Calculated effective magnetic moment µ eff (calc) of rare-earth ions,... | Download Scientific Diagram